PNDT- Compliant Technology and Corporate Reforms in Indian Ultrasound Devices Market
Author: Swapna Supekar | January 06, 2017
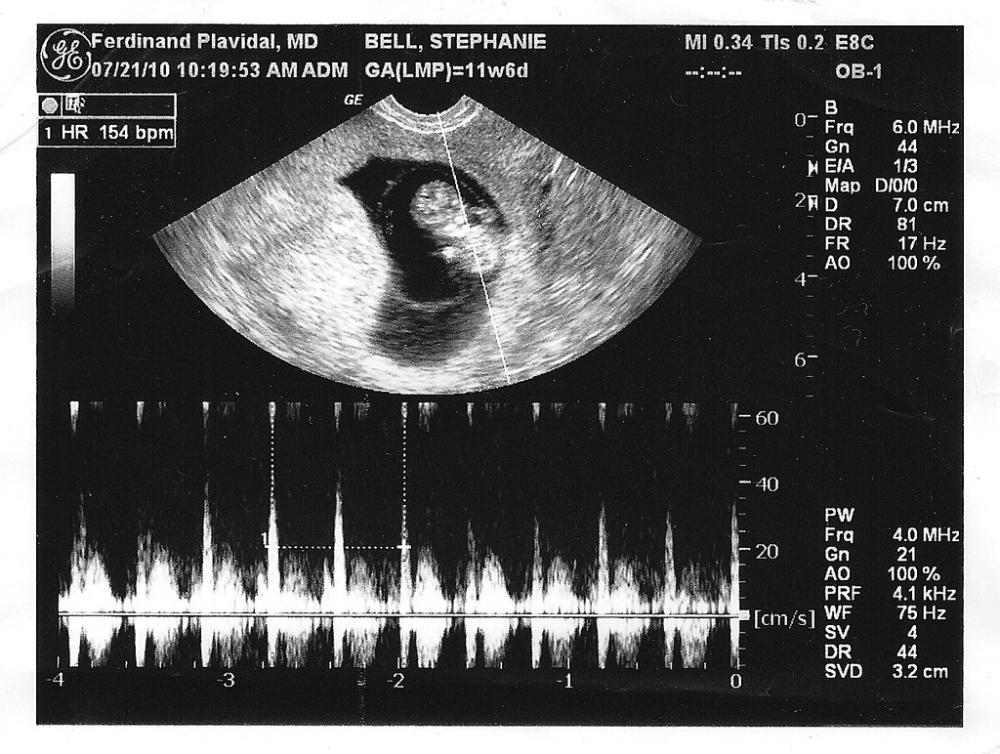
The distribution of compact and reliable portable ultrasound devices pan India was supposed to be pathbreaking move. Especially, for the 68.8% of people who reside in rural regions with low-key access to healthcare diagnostic services and point-of-care devices are their only recluse. A couple of years back, the trend for "on-call" portable ultrasound machines spread like wildfire throughout the country.
The Union health ministry later found out the dark truth shrouded beneath the spreading network of devices. It pointed out how this trend was a direct violation of the Pre-conception and Pre-Natal Diagnostic Techniques Act (PC & PNDT Act), 1994. Under this act of law, the sex determination of an unborn child was made illegal, with exceptions for the diagnoses of specified sex-related diseases. The PNDT act made allowance for the portability of an ultrasound devices within the premises of a healthcare center such as a hospital or clinic.
The ministry later went ahead to draft a proposal that would implement ban over the use of “on-call” ultrasound devices.The proposal was presented in front of the Central Supervisory Board (CSB) meeting in 2012 and duly brought into effect over due course of time.
Corporate Responsibility
Less than a decade after the PNDT Act came into force, amendments were made to identify the responsibilities of the manufacturers in the ultrasound devices market. These policy reforms enforced the vendors to keep a check on their customers if they were PNDT certified. However, a leading global company, GE Healthcare (GEHC) identified that “[the] laws are weakly enforced by the government” and the technology in itself was not the root cause of the issue.
The GEHC India division resolved to take care of the situation under its corporate social responsibility (CSR). It worked to increase the safety mechanisms against the illicit sales of ultrasound devices and extend support for the awareness campaigns regarding the human rights that were endangered by the illegal practice. The company’s initiative was exemplary of the ideal course of conduct for corporate players. It established a reference for the other companies to plan commercial strategies such that their products were being distributed in the specified channels
Tagging Along technical Solutions
The Himachal Pradesh State government’s decision to implement the Mukhyamantri State Healthcare Scheme early in 2015 was a welcome event among the state population. It outlined the ruling government’s intention to ease the access of healthcare services for its people. Exactly a year later, the state health minister Kaul Singh made news when he called out for the installation of Active Tracking Devices in each government-owned and private ultrasound device across the state. The minister also instructed the employment of adequately qualified i.e., diploma or degree holders, to operate these machines. He added the intentions to spread large-scale awareness campaigns regarding female feticide with the help of NGO and Asha workers.
The decision was in retaliation to the drastic drop observed in sex ratio in the region. This was a direct indication that despite the strict legislation were in no way delivering expected results. Apparently, monetary rewards for those reporting the case of pre-natal sex determination was not working as well. Thus, the slight yet smart technical addition is being made to the ultrasound devices. The initial plan is to start a pilot project across three districts – Kangra, Hamirpur, and Una. During later stages, the plan would be executed in several phases across the other districts in the state.
This initiative brings a positive light of hope for the ultrasound devices market that faces considerable retarding force from the Pre-Natal Diagnostic Act, when it comes to the seamless distribution of portable types.
According to a research report, the global ultrasound devices market is estimated to garner revenue worth $10,476 million by the end of 2022. At present the industry size in India is worth 148$ million. Expert analysts suggest that the industry is expected to register a CAGR of 9.6% during 2012–2019. Customized healthcare schemes for women bring more business to the companies operating in the region. The industry expects to see growth in business regarding the changing policy reforms by the current ruling government. Get detailed information visit: https://www.alliedmarketresearch.com/ultrasound-devices-market
*****




.jpg)